Clinical Findings Associated with EEHV Hemorrhagic Disease in Elephants
Lauren L. Howard, DVM, Dipl. ACZM
Associate Veterinarian, Houston Zoo
General Introduction:
EEHV Hemorrhagic Disease (EEHV HD) can have a rapid onset and progression. Animals have died within 10 hours of the first observation of clinical signs. In at least three of the animals that survived EEHV HD, clinical signs generally worsened for 1-2 days after the initiation of therapy and slowly dissipated over the course of 10-15 days. EEHV HD most commonly affects Asian elephants between 1 and 8 years of age. Clinical disease has been observed in Asian elephants older than 8 years, and in African elephants, but less frequently.
Initial observations in an elephant ill from EEHV HD are often very vague and can easily be attributed to other conditions. In most cases the first observation is lethargy, decreased appetite, or both. It is tempting to attribute decreased activity level or lameness to a bout of rough play, or decrease in food or water consumption to a recent tusk eruption or mild colic. It is imperative that animal care staff be alert for any changes in an elephant calf’s condition and to assume illness is due to EEHV HD until proven otherwise.
Training Observations:
One behavior observed by elephant managers in elephant calves ill from EEHV HD is that the calf demonstrates less compliance than usual during training sessions. It has also been observed that calves ill from EEHV HD are more attached to the herd and more reluctant to separate from the group than usual.
Clinical Observations:
The following signs have been observed in elephants ill from EEHV HD. The presence of more than 1 or 2 categories of signs makes EEHV more likely, and treatment should be initiated and continued until PCR results are available:
- Decrease in food or water intake.
- Alteration in sleeping pattern or activity: more than 20% change in activity level from baseline.
- a. This can mean sleeping more OR sleeping less, as some calves appear reluctant to lay down when they are ill.
- Signs of discomfort or acute pain (either abdominal or musculoskeletal)
- a. Abdominal: stretching, rolling
- b. Musculoskeletal: Lameness/stiffness
- Changes in fecal output:
- a. diarrhea, decreased stool production, hard stools, or constipation
- Mentation change:
- a. elephant appears confused or is demonstrating other neurologic signs
- Oral lesions:
- a. hyperemic or dark, red, oral mucosa
- b. vesicle formation or ulceration
- c. petechiae/ecchymoses
- Ocular abnormalities:
- a. scleral injection
- b. icterus of sclera
- c. retinal hemorrhage
The presence of edema or cyanosis, even if not accompanied by any of the above clinical signs, should warrant initiation of treatment immediately:
**Edema:
- swelling or fluid accumulation visible grossly
- particularly head, trunk, neck
- this excludes the presence of ventral or dependent edema, which is considered a non-specific finding associated with many etiologies in elephants
**Cyanosis: present on tongue or other mucus membranes
Physiologic Parameters:
Physical examination of an elephant suspected to be ill from EEHV HD may reveal the following abnormalities:
- Cardiac abnormalities as noted on physical examination: (must know elephant’s baseline information)
- a. Tachycardia
- b. Arrhythmia
- c. Heart murmur
- d. Changes in blood pressure (must know elephant’s baseline information)
- e. Pulse oximetry < 95% (as measured on the lip)
- Alteration in body temperature (above or below normal, must know elephant’s baseline) as measured by fresh fecal bolus temperature or “life chip” microchip body temperature.
- Evidence of fluid accumulation as noted on ultrasound examination:
- a. Abdominal fluid
- b. Pericardial fluid
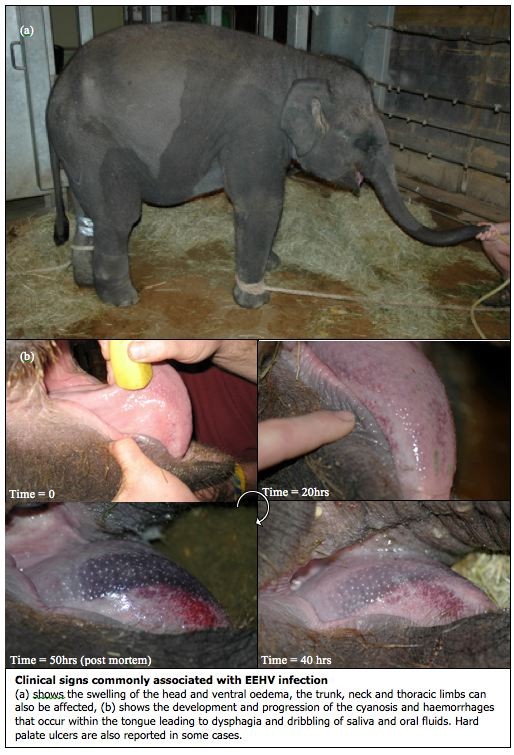
Clinical Pathology:
Evaluation of the complete blood cell count (CBC) in an elephant suspected of being ill from EEHV HD can yield very helpful information. It is most helpful when the normal ranges of the CBC values are known for the individual elephant in question. The presence of any of the abnormalities listed below is indication that an elephant should be started on EEHV therapy until PCR results are available:
Anemia and thrombocytopenia (generally speaking, 20% or more decrease from normal values) have been observed in cases of EEHV HD. Often, a rebound thrombocytosis will follow the initial thrombocytopenia.
The presence of any immature neutrophils/heterophils (“bands”) on a blood smear is not normal in elephants; this is clinically significant and indicates a serious disease process, whether EEHV HD or another condition.
Typically, the most abundant white blood cell in elephant CBCs is the monocyte. In cases of acute EEHV HD, as well as in other infections, neutrophils/heterophils may outnumber the monocytes and become the predominant cell type. This is abnormal and should be considered significant. In cases of clinical EEHV associated illness at the Houston Zoo, the total WBC of Asian elephants ill from EEHV 1, 4 and 5 dropped significantly just before, or at the same time as, viremia increased significantly. The presence of a decreased WBC, a decrease in absolute monocyte number, or predominance of neutrophils, in an elephant with any degree of EEHV viremia should be considered significant, and therapy should be initiated and continued until PCR results are available. In several recent, closely monitored cases of EEHV-associated illness, the total WBC was found to be decreased for one to five days, then started to increase, and was followed by a rebound leukocytosis for several days or weeks.
In our clinical observations at the Houston Zoo, serial monitoring of whole blood EEHV PCR and of the WBC in an elephant affected by EEHV HD provided a very useful tool for gauging decisions on extent and duration of therapy.
Evaluation of an elephant’s serum biochemistry profile is important for assessing the overall health of the animal, but to date we have not identified any significant biochemical abnormalities that specifically indicate illness associated with EEHV HD.
Increase of an acute phase protein, serum amyloid A (SAA), has been associated with EEHV-1 viremia over 10,000 vge/ml in Asian elephants. The Avian and Wildlife Laboratory of the Comparative Pathology Lab at the University of Miami (http://cpl.med.miami.edu/avian-and-wildlife/) will test serum for SAA.
Determination of coagulation status and clotting times should be considered in elephants suspected of illness associated with EEHV HD. Normal coagulation profiles of the Asian elephant have been established; however alterations in these values during EEHV have not been evaluated.
Molecular Diagnostics:
The gold standard for diagnosis of illness associated with EEHV HD is polymerase chain reaction (PCR) of a whole blood sample for EEHV DNA. In North America, EEHV-1 is the most common EEHV species associated with EEHV HD in Asian elephants, and should be the first virus tested for in a sick elephant. Less frequently, EEHV-4 and EEHV-5 have been associated with clinical illness in Asian elephants, and EEHV-2 and EEHV-3 have been associated with illness in African elephants. The National Elephant Herpesvirus Laboratory and Baylor College of Medicine have the capability to test for all known EEHV viruses (click here to see list of EEHV testing laboratories).
Through routine monitoring of Asian elephant calves in several North American institutions, we have learned that some degree of EEHV viremia can be detected in clinically healthy elephants. Typically these viremias are at or just above the detection limit of the qPCR test. Therefore, the presence of EEHV viremia alone, particularly in a subclinical elephant, is not in itself confirmation of illness from EEHV HD. In the case of a viremic elephant that has normal CBC results and no abnormal clinical findings, serial monitoring of the viral loads (and close physical observation of the elephant) is recommended to determine if the viremia is increasing. It has been observed that clinical signs develop when EEHV-1 viremia approaches 10,000 vge/ml; however we have not evaluated enough cases to make definite conclusions. The trend of the viremic load is more important than the absolute number, with a rapid increase in viremia indicating a need for more intensive therapy and closer monitoring.
Trunk washes also can be submitted for EEHV PCR (click here to see list of EEHV testing laboratories). Following a viremia or re-activation event, it has been observed that elephants will secrete large amounts of EEHV DNA from their trunks for several weeks. Trunk wash fluid can be centrifuged and the pelleted material evaluated for EEHV DNA via PCR. This can confirm shedding of EEHV and can be epidemiologically useful, but does not provide any clinically significant information that would help in diagnosing illness associated with EEHV HD.
Thank you to Angela Fuery of Baylor College of Medicine; Joe Flanagan, Maryanne Tocidlowski and Maud Marin of Houston Zoo; and Ellen Wiedner, Point Defiance Zoo & Aquarium for reviewing this prior to submission.
Selected References:
Atkins L, JC Zong, J Tan, A Mejia, SY Heaggans, SA Nofs, JJ Stanton, JP Flanagan, L Howard, E Latimer, MR Stevens, DS Hoffman, GS Hayward, and PD Ling. 2013. Elephant endotheliotropic herpesvirus 5, a newly recognized elephant herpesvirus associated with clinical and subclinical infections in captive Asian elephants. J Zoo Wildl Med 44(1): 136-134.
Gentry PA, ML Ross, and M Yamada: Blood coagulation profile of the Asian elephant (Elephas maximus) In Gentry PA, Ross ML, Yamada M: Zoo biology, Vol 15, New York, 1996, Wiley-Liss, pp 413-423.
Isaza R, E Wiedner, S Hiser and C Cray. 2014. Reference intervals for acute phase protein and serum protein electrophoresis values in captive Asian elephants (Elephas maximus). J Vet Diagn Invest26(5), 616-621.
Stanton JJ, C Cray, M Rodriguez, KL Arheart, PD Ling, and A Herron. 2013. Acute phase protein expression during elephant endotheliotropic herpesvirus-1 viremia in Asian elephants (Elephas maximus). J Zoo Wildl Med 44(3): 605-612.
Stanton J, JC Zong, C Eng, L Howard, J Flanagan, M Stevens, D Schmitt, E Wiedner, D Graham, RE Junge, MA Weber, M Fischer, A Mejia, J Tan, E Latimer, A Herron, GS Hayward, and PD Ling. 2013. Kinetics of viral loads and genotypic analysis of elephant endotheliotropic herpesvirus-1 infection in captive Asian elephants. J Zoo Wildl Med 44(1): 42-54.
Wiedner E, L Howard and R Isaza. 2012. Treatment of elephant endotheliotrophic herpesvirus (EEHV). In: Fowler, ME and RE Miller (eds) Zoo and Wild Animal Medicine, 7th ed: 537-543.


